Resonance Health provides comprehensive clinical trial services for pharmaceutical companies using imaging end points in their Phase II, III, and IV clinical trials. A fully compliant web based infrastructure enables image data and results to be securely transferred from anywhere in the world to and from our core lab in Australia. Resonance Health provides objective, reproducible, and quantified measurements in accordance with our unique Quality Assured Service Delivery model.
Resonance Health’s expertise in the provision of imaging core lab servicesfor pharmaceutical company clinical trials has been utilised in over 20 multicenter studies spanning 25 countries over 10 years. Imaging biomarkers are increasingly used in clinical studies providing a safe, non-invasive alternative to invasive procedures such as liver biopsy. Imaging provides an ideal solution where repeat measurements are required over the life of the study.
Resonance Health has been involved in clinical studies where imaging has been used to assist in:
- The assessment of subject inclusion or exclusion
- Supporting primary and secondary end-points demonstrating drug efficacy
- Evaluating the safety profile of a new therapy
Overview of Services
ISO 13485 certified centralised Quantitative Image Analyses, available globally, with regulatory clearances in the US (FDA), Australia (TGA), and Europe (CE Mark):
o FerriScan® R2-MRI– internationally recognised as the gold standard in liver iron concentration (LIC) measurement
o Cardiac T2* for Iron Assessment – offered as a dual analysis service with FerriScan to provide more comprehensive information regarding body iron stores
o HepaFat-Scan® – volumetric liver fat fraction (VLFF)for quantitative measurement of hepatic steatosis
- Quantitative Image Analysis available globally in an investigational setting:
o Bone Marrow R2 for Iron Assessment – provides an estimation of iron levels in the bone marrow – approval from TGA, CE mark & FDA is expected by end of 2017.
o Quantitative Iron Assessment in Other Organs – surrogate iron measurements (R2 / R2*) in other organsincludingpancreas, spleen, and kidney
o Pancreatic Fat Assessment– quantitative assessment of pancreatic fat
o Visceral / Subcutaneous Fat and Organ Fat in Metabolic Disease – quantitative assessments of visceral fat, subcutaneous fat, epicardial fat
o Fibrosis and Inflammation – a combination of MRI measures to assess liver fibrosis and inflammation
o Liver Biopsy – Stereology Services– quantitative assessment of hepatic steatosis of digitised biopsiesusing stereology
o Organ Volume Measurements – measurements of various organs such as the liver and spleen
o Other – customised imaging solutions
- Scanner verification using phantoms (in accordance with the FDA Draft Guidance Clinical Trial Imaging Endpoints Process Standard, March 2015)
- Secure 21 CFR Part 11 compliant web-based infrastructure for image transfer and reporting
- Imaging trial design consultancy
- Core Laboratory Services including project and data management
The Resonance Health team includes talented scientists, researchers, and academics from across the globe. Our specially trained MRI physicists are experts in image analysis using our patented world leading technologies, and are supported by the wider research and development team at the forefront of imaging analysis development. Resonance Health also partners with many other prestigious organisations, including government research bodies, universities, international patient organisations, and centres of excellence to develop and provide world leading solutions to the clinical and pharmaceutical communities.
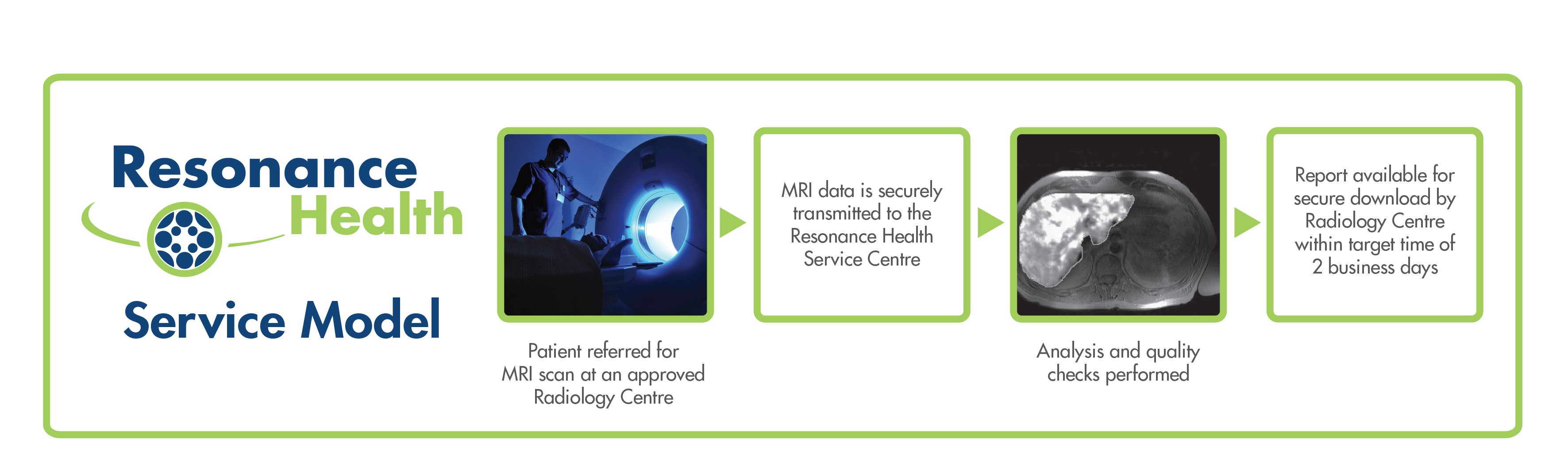
Imaging Analysis Services
Image data is securely transferred to Resonance Health’s central ISO certified Service Centre, where it is analysed by our team of experts. Result reports are made available for download by authorised personnel.
FerriScan®R2-MRI– MRI measurement of liver iron concentration
FerriScan R2-MRI is a proprietary, patent-protected imaging technology for measuring liver iron concentration (LIC) and is the first non-invasive test for LIC to have achieved regulatory clearance and approval in the US, Europe, and Australia. FerriScan is internationally recognised as the gold standard in liver iron concentration measurement and offers multiple significant advantages over other methods of obtaining LIC including:
- Extensive validation against liver biopsy across multiple centres and multiple MRI scanner makes and models from very low to very high iron levels
- The FerriScan LIC measurement is unaffected by the presence of fibrosis and fat
- A standardised approach to MRI centre set up, data acquisition, and analysis
- ‘Free breathing’ data acquisition suitable for paediatric patients
- FerriScan has FDA, TGA and CE Mark regulatory clearance for the measurement of LIC and is the only MRI test for LIC that gained an additional level of clearance from the FDA as a ‘companion diagnostic in the NTDT setting’
- Centralised image analysis in accordance with rigorous Quality Management System standard operating procedures to ensure accuracy and reproducibility of the results
- Verification of each and every scanner using a FerriScan Phantom Pack to confirm the correct scanner configuration as an added quality control and standardisation measure.
Cardiac T2*– MRI Assessment of Iron in the Heart
Resonance Health offers a dual analysis service including Cardiac T2* measurement in addition to a FerriScan to provide more comprehensive information regarding body iron stores. Resonance Health’s Cardiac T2* analysis service has regulatory approval in the USA, Australia, and Europe. The Cardiac T2* analysis service is available to suitably equipped MRI Centres internationally.
Bone Marrow R2-MRI– MRI Assessment of Iron in the Bone Marrow
Resonance Health is able to non-invasively assess bone marrow iron levels using FerriScan images. Elevated bone marrow iron in potential bone marrow transplant recipients is associated with a range of poorer health outcomes post-transplant. Quantitative assessment of bone marrow iron pre-transplant may help to predict patient prognosis and improve patient outcomes. The current gold-standard for measuring bone marrow iron is histopathological grading, which is semi-quantitative, non-standardised and subject to inter-observer error. Bone marrow R2-MRI is non-invasive, quantitative, and correlates strongly with iron in bone marrow.
Quantitative Iron Assessment in Other Organs
Resonance Health has experience in measuring surrogate iron measurements (R2 / R2*) in organs including pancreas, spleen, and kidney.
HepaFat-Scan -MRI measurement of Volumetric liver Fat Fraction (VLFF)
HepaFat-Scan is a proprietary, patent-protected imaging technology for measuring liver steatosis and has regulatory clearance and approval for clinical use in the US, Europe, and Australia. HepaFat-Scan reports a percentage volumetric liver fat fraction (VLFF) and has been validated against liver biopsy. The HepaFat-Scan VLFF can be converted to a NASH CRN liver steatosis grading.
HepaFat-Scan offers multiple advantages over other MRI methods:
- Validated against liver biopsy across multiple MRI scanner makes and models from very low to very high liver fat levels in non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and autoimmune hepatitis.
- Standardised approach to MRI centre set up, data acquisition, and analysis
- Single breath-hold data acquisition
- HepaFat-Scan has FDA, TGA and CE Mark regulatory clearance for the quantitative assessment of hepatic steatosis
- While standard imaging analysis utilises two regions of interest (ROI), multiple ROI are available upon request
- Centralised image analysis in accordance with rigorous standard operating procedures to ensure accuracy and reproducibility of the results
Liver Biopsy – Stereology Services
Resonance Health can provide a quantitative assessment of hepatic steatosis of digitised biopsies using stereology. Stereology offers multiple key advantages:
- Stereology is a non-biased method that delivers a standardised assessment of steatosis from biopsies. In particular, this can add value in circumstances where more than one histopathologist is engaged across multiple study sites. As histopathological scoring for a given biopsy can vary between experts, stereology can provide a means to ascertain the extent of bias.
- Stereology can be applied retrospectively to analyse archived biopsy data originating from completed studies so as to determine if a ‘drug effect’ has been obscured because of disparate assessments from expert histopathologists.
- As stereology is used to determine the number of hepatocytes with fat vesicles (macrovesicular/microvesicular steatosis) within a liver biopsy, its findings are reported as a volumetric liver fat fraction (VLFF), and can be directly compared to the HepaFat-Scan measurements.
It should be noted that stereology can potentially be applied to the non-biased assessment of other disease related markers, including fibrosis, hepatocyte ballooning, and neutrophil infiltration. In view of the potential for the unbiased assessment of these clinically important biomarkers of liver disease, Resonance Health are currently pursuing methodologies to optimise this process.
Visceral, Subcutaneous, and Organ Fat in Metabolic Disease
A hallmark in the establishment of metabolic disease is the deposition of abnormal fat within and around organs and muscle tissue. In view of this, Resonance Health have developed imaging modalities that can provide quantitative assessments of visceral fat, subcutaneous fat, and epicardial fat. Such data can be used for the purposes of natural history studies, baseline assessments, and for the monitoring of drug-related effects. Given the links between hepatic steatosis, diabetes, and non-alcoholic steatohepatitis, Resonance Health have developed a number of investigational imaging tools to quantify the presence of fat within tissues such as pancreas, kidney, and skeletal muscle. These services can be scoped to meet individual requirements.
Organ Volume Measurements
Volume measurements of various organs such as liver and spleen for use in identifying disease states or tracking change over time as result of treatment.
Research Services and Collaborations
In recognising the changing needs of the clinical community and healthcare industry, Resonance Health remains committed to the development of novel, clinically relevant imaging modalities. As such, our team of scientists and academics are available to assist our pharmaceutical partners in the development of customised imaging solutions to maximise the return on their clinical trial investment.
Core Laboratory Services
Resonance Health provides quality assured imaging analysis services and customisable project and data management services. This supports our clinical trial customers meet trial protocols and requirements stipulated by ICH-GCP and international regulatory authorities. Our project and data management services involve the provision of dedicated expert resources for trial management including the setup and management of MRI Centres, data management, reporting, and issue resolution. Pharmaceutical collaborators benefit from this full service to ensure operational excellence in the delivery and management of their studies. We strongly recommend project and data management alongside the provision of the analysis services.
Quality Assured Imaging Analysis Services
Resonance Health has extensive quality assurance processes and procedures to ensure compliance with regulatory requirements, FDA 21 CFR Part 820 and international standards certifications ISO 13485. These cover every aspect of the centralised image analysis services for clinical trials, including a quality control review of all image data, quantitative image analysis, a second independent read of all image analysis, quality assurance checks on every result, and a full audit trail.
Resonance Health has invested significant resources in the development of the HIPAA and HITECH compliant FAST system; a web-portal for the secure transmission of image data and patient results from anywhere in the world. FAST has a number of stringent physical and electronic security measures and Resonance Health employs a comprehensive set of policies and procedures to ensure protected health information is secure.
Project Management Services
- Development of an Imaging charter and other study specific documentation
- Establishing study specific project management files and communication plans
- Imaging Centre selection, training, and management to ensure standardisation of imaging
- Central point of contact for reporting and issue escalation
- Enforcing protocol patient identifier anonymity, including data query communications.
- Trial specific standard operating procedures to ensure trial protocol requirements are met and Resonance Health staff members are trained on specific requirements.
- Dedicated internal auditing of the trial records and data management.
- Provision of trial specific documentation as required. Protocol specific instructions can be added to the Result Reports if required (e.g. a statement added to the report indicating dosage instructions).
- Compliance with all trial related regulatory requirements (in addition to ISO 13485, FDA 21 CFR Part 820 and the EU MDD), including 21 CFR Part 11, HIPAA and HITECH Acts, ICH Guidelines, EU Data Protection Directive and Safe Harbor provisions.
- Accepting and in full support of regulatory authority and trial vendor audit requirements, including desktop audits, conference calls and in-person inspections of the Resonance Health quality system and services.
- Full Design Control and Validation of ancillary processes and systems required for service delivery for clinical trials and record retention requirements.
- Record Retention and Storage: Maintenance of electronic and hardcopy (under lock and key) trial related records in dedicated secure locations until trial completion. Resonance Health has validated systems for storage and backup of all source data in accordance with the FDA draft guidance titled Clinical Trial Imaging Endpoints Process Standard (March 2015). All hardcopy and electronic subcontractor processes for archiving and backup are governed by a Business Associate Agreement compiled to meet ICH-GCP, HIPAA and HITECH Act requirements.
Data Management Services
- Establishment of a data transfer specification and database.
- Electronic image data tracking and management
- Blinded or un-blinded reporting of results to CRO and data cleaning as required.
- Electronic imaging tracking (using Resonance Health’s secure online tracking system – FAST).