
FerriScan® now on 3T MRI machines
We are pleased to advise our customers that FerriScan®, our flagship product, is now available for use on 3 tesla (3T) MRI scanning machines.
FerriScan® is internationally recognised as the gold standard for the assessment of liver-iron-concentration (LIC) for patients with iron overload diseases. This MRI-based technique is non-invasive and eliminates liver biopsies, and is now recommended in clinical patient management guidelines and international standards of care. Since our inception, we have completed over 65,000 FerriScan® patient reports to assist patients suffering iron overload disorders around the world. Until now, FerriScan® has only been available at MRI facilities with 1.5 tesla (1.5T) field strength MRI machines.
The Company commenced studies several years ago, aimed at adapting the FerriScan® acquisition protocol from 1.5T to 3T MRI scanners, allowing for better usability of the FerriScan® service across a broader range of MRI machines and global sites. We are pleased to advise that we recently finalised the calibration of the FerriScan® protocol, with FerriScan® now commercially available for use in clinical trials and in the routine clinical management of patients, on both 1.5T and 3T MRI machines.
3T MRI machines offer advantages for clinicians and patients, including shorter scan times and greater image contrast compared with 1.5T MRI machines, and are growing in prevalence around the world. In the United States, 1.5T and 3T scanners are now being purchased in approximately equal numbers and dominate new sales, with 3T scanners already representing approx. 15% to 18% of total MRI scanners in Europe and North America.[i]
We are committed to ensuring our services – including FerriScan® – remain at the forefront of technical development and are available to as many patients as possible, so that their diseases can be managed effectively. Ensuring that FerriScan® is available on as many MRI machines as possible is also a key element of our strategy to grow our clinical trial services contracts and revenue. Approximately 38% of our revenue is derived from providing analysis and other services to pharmaceutical companies undertaking clinical trials for new treatments of iron overload and other diseases. For more on FerriScan click here
Work also continues to progress the adaptation of FerriSmart®, our AI-assisted evolution of FerriScan®, to 3T MRI scanning machines. This will further enhance availability and convenience for clinicians and patients in large new markets. For more on FerriSmart® click here
[i] Could Very Low Field Strength Be the Next Frontier for MRI?, Burhan Ahmed Khan, M.D., Hyperfine intern, Eliot L. Siegel, M.D., Associate Vice Chair, University of Maryland School of Medicine, Diagnostic Imaging, 11 March 2021

Cardiac T2* on 3T scanners (for clinical trials)
Cardiac T2* is now also available on 3T scanners, although at this stage only for our Pharma customers engaged in clinical trials, and for investigational uses. This upgrade was driven by our clinical trial customer demand, with some trial sites only having 3T scanner capability.
Work is underway to make this upgrade available to all our clinician customers and we are aiming to have this completed in the next 6 months. 3T MRI machines offer advantages for clinicians and patients, including greater image contrast compared with 1.5T MRI machines.
Resonance Health is committed to being at the forefront of technical development, which includes being calibrated on new generation MRI machines, enabling service availability to as many patients as possible, so their diseases can be managed effectively by physicians.
For more on Cardiac T2* click here

HepaFat-Scan® now with PDFF + steatosis
HepaFat-Scan® has been updated and in the coming weeks patient reports will also include proton-density-fat-fraction (PDFF) and a steatosis grading, adding to the usability of this product, particularly for clinical trials needing scanner-agnostic standardised analysis.
HepaFat-Scan® is the only liver fat assessment that reports volumetric-fat-fraction (VLFF) which is the only approach calibrated against biopsy and the only validated for use with children. PDFF together with VLFF provides the most comprehensive liver fat analysis.
For more information about HepaFat-Scan® please click here

Upgrading of HepaFat-AI®
Concurrent with the upgrade of HepaFat-Scan®, our FDA cleared + CE Marked HepaFat-AI® device has been improved and will soon be submitted to the FDA under a special 510(k) application. The upgraded service will be available to customers in the coming months.
The new version of HepaFat-AI® includes a new liver segmentation module and additional neural network (AI) training has been completed showing improved performance. A PDFF score and a steatosis grade are already regulatory cleared components of HepaFat-AI®.
For more information about HepaFat-AI® please click here

Shorter acquisition FerriScan® + FerriSmart®
Work has continued on validating a much shorter MRI imaging sequence for FerriScan® and FerriSmart® with a 75% targeted reduction in patient MRI scanner time of 1.5 minutes (90 seconds – 5 breath-holds) versus the current MRI sequence time of 8-10 minutes.
This has now progressed successfully through the proof-of-concept stage, with datasets now being acquired to complete our stringent quality and regulatory validations. This will vastly improve patient experience and increase scanner throughput at imaging centres.

Other device innovations - Cardiac T2*-AI
Other projects advancing include development of an AI-assisted version of our regulatory cleared Cardiac-T2* device, to complement our three existing regulatory cleared AI-assisted (fast-turnaround) devices; FerriSmart®, HepaFat-AI®, and LiverSmart®.
CardiacT2*-AI will provide patient reports in seconds to clinicians assessing cardiac-iron levels, at an affordable price-point. This has been identified by WHO related Cyprus-based, Thalassaemia International Federation (TIF), as critical in large markets where iron-overload diseases are prevalent.
In 2021 Resonance Health signed a Patient Access to FerriSmart® Letter of Agreement (LoA) with TIF (FerriSmart Access Initiative) for the deployment of FerriSmart® vouchers, provided by us at no cost, across low-and-middle income countries in Asia, West Pacific, and Europe. TIF is engaging with National Thalassaemia Associations and physicians to facilitate utilisation of FerriSmart®, and with national health authorities and key opinion leaders (KOLs) on the value and cost effectiveness of integrating regular iron-load monitoring into clinical management protocols (establishing local standards of care). Executive Director of TIF, Dr. Androulla Eleftheriou, said the following of the new agreement:
“TIF’s vision is to ensure equal access to quality healthcare for every patient with thalassaemia and other haemoglobin disorders across the world. Iron chelation is one of the two cornerstones of thalassaemia management (the other being blood transfusion) and accurate iron load monitoring of the heart and liver contributes immensely to the assessment of the effectiveness of chelation therapy. Thus, the development of iron-related complications is prevented while at the same time allowing for better resource distribution for healthcare system improvements and developments.
To that end, we are delighted to reach this important agreement with Resonance Health for the rollout of FerriSmart® into low-and-middle income countries, starting with nations in Asia, West Pacific, and Europe. Resonance Health’s FerriScan® product is the only global gold standard to-date for measuring liver-iron-concentrate (LIC) which is critical to managing thalassaemia. While many of the developed world’s reference centres and KOLs have adapted FerriScan®, its application in the more thalassaemia-prone low-and-middle income countries has been severely hindered mainly due to FerriScan’s® reliance on highly trained personnel, which adds significantly to its cost and renders it unaffordable and unscalable in many lower income regions.
We are thus thrilled with Resonance Health’s development of FerriSmart®, a new automated AI product calibrated against the FerriScan® gold standard and now available for patients globally. FerriSmart® uses the same non-invasive R2 MRI approach as FerriScan®, which TIF acknowledges its contribution as the most accurate and safest LIC diagnosis, and which has been thoroughly used in clinical trials for reliable iron measurements. To that end, in 2017 TIF released a global alert cautioning clinicians from using untested, non-standardized methods of iron assessment, which can be highly unreliable particularly at elevated iron levels and may jeopardize the health of the patients.
FerriSmart® is a breakthrough for Thalassaemia patients in low-and-middle income countries where thalassaemia is unfortunately and sadly often sub-optimally managed and the inability to accurately assess LIC contributes to this. Committed to improving the lives of patients globally, I am delighted to see the conclusion of this agreement with Resonance Health as a great step forward for better health outcomes of thalassaemia patients and we hope this is the first step in a multi-year sustained rollout of FerriSmart®. Moreover, TIF looks forward to the development and approval of additional iron load monitoring tools that will be of equal reliability and quality, and that will meet the approval processes of the EMA and FDA so as patients and healthcare professionals will have the opportunity to choose the tool they wish or prefer to use.”
Managing Director of Resonance Health, Mitchell Wells, said the following of the new agreement:
“Resonance Health has great admiration of TIF and the work it does improving the lives of patients with thalassaemia. We are delighted to partner with them on the deployment of FerriSmart® into new markets where our device is needed. TIF has the experience and contacts in these markets to accelerate the uptake in adoption and usage of our new AI devices. Our immediate objective is to ensure that patients and clinicians across the world learn of FerriSmart® and have an opportunity to use it and benefit from it. We are proud to support TIF in this initiative and we are positioned to do so with our enhanced sales and marketing resources.”

Non-invasive liver fibrosis invention
Resonance Health has lodged a provisional patent application for an invention to detect and assess fibrosis in the liver and other organs, utilising non-invasive magnetic resonance imaging (MRI).
Chronic liver disease – a global epidemic
Chronic liver disease is a leading cause of death worldwide, incorporating a wide range of prevalent human diseases including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). These two diseases are estimated to affect one-quarter of the world, or approximately 2 billion people.[i] If the prevalence of NAFLD continues to rise in line with the global obesity epidemic, the healthcare burden of NAFLD over the next 10 years could increase to $1.005 trillion in the USA alone.[ii]
Additionally, viral hepatitis, which is mostly incurable and imparts a high disease burden globally, is estimated to affect 257 million and 71 million people living with hepatitis B virus (HBV) and hepatitis C virus (HCV) infections respectively.[iii][iv]
Despite the significant disease burden, liver disease remains on an upward trajectory with clinicians citing an explosion of fatty liver-related health complications globally and lamenting the lack of validated techniques to enable earlier intervention.[v]
Liver fibrosis and chronic liver disease
Liver fibrosis, or scarring, occurs in and is caused by most types of chronic liver disease. Liver fibrosis is typically progressive and insidious in nature and may not cause obvious symptoms early in its onset.
Once fibrosis progresses to cirrhosis (an advanced form of liver fibrosis) it typically gives rise to greatly diminished liver function with major associated negative health impacts and, ultimately, organ failure that requires a liver transplant as the only available treatment. Liver cirrhosis is a main cause of death worldwide, and a leading cause of disability -adjusted life years.[vi]
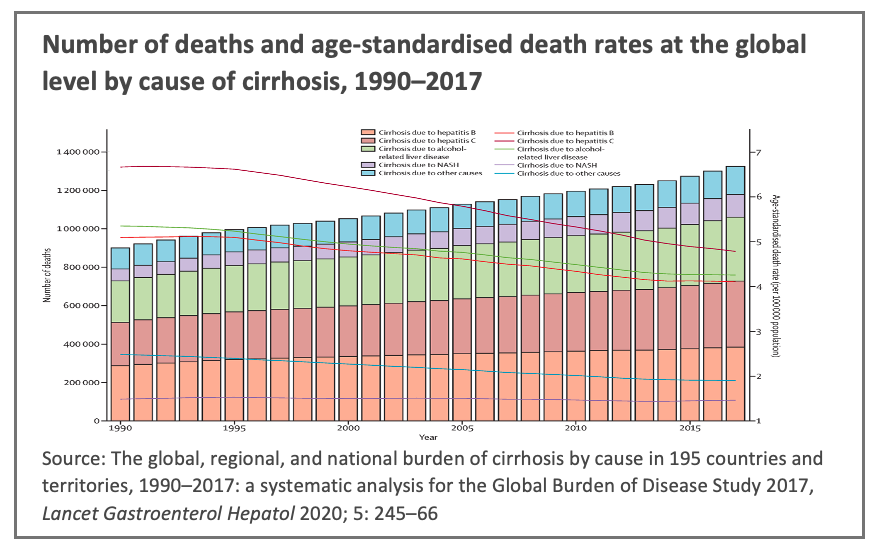
A growing body of clinical evidence indicates that liver fibrosis is reversable after eradication of the underlying causal liver disease, and it further suggests that early detection and assessment of liver fibrosis is essential to reversing its progression.
The development of drugs that seek to address chronic liver diseases such as NASH and NAFLD and to reverse the associated fibrosis is a highly active area of research by global pharmaceutical companies. Accurate assessment of liver fibrosis is a critical endpoint for such trials to assess the performance of their drugs.
Assessment of liver fibrosis – biopsy the current gold-standard
Fibrosis staging is reported using established and standardised scoring systems that reflect the expansion of fibrotic tissue across the liver architecture – such as the METAVIR scoring system which reports the progression of the disease based on staging from F1 (no fibrosis) through to F4 (cirrhosis).
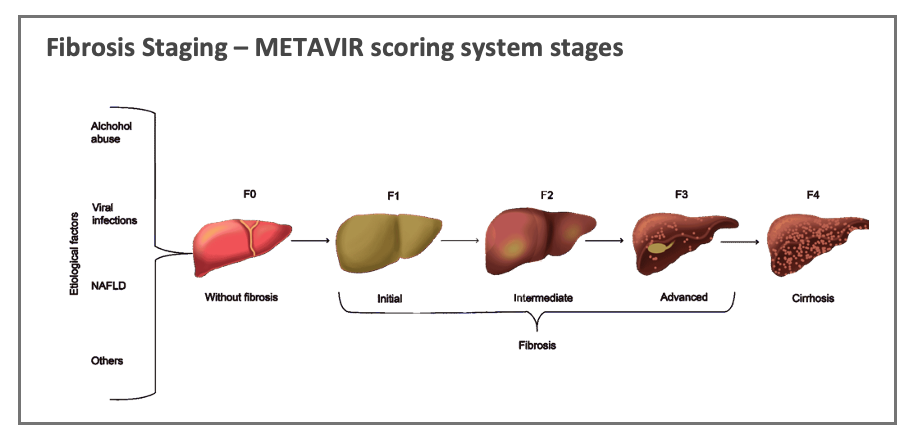
The current global gold-standard for determining the presence of liver fibrosis and its progression is by liver biopsy which involves the collection of liver tissue sample(s) from the patient using a needle, which is then analysed by a pathologist.
Liver biopsies are not trivial procedures; they are invasive and can give rise to significant side effects including pain, bleeding, infection, hospitalisation, and even death. The biopsy procedure is slow and expensive as it requires the involvement of multiple medical specialists and facilities.
Liver biopsies are also subject to significant sampling error because they only assess a minute fraction of the liver organ and are unavailable to patients with various comorbidity factors including blood clotting conditions and infections.
Demand for non-invasive approaches for clinical trials
For pharmaceutical companies undertaking clinical trials, the invasive and risky nature of liver biopsies presents a significant obstacle to obtaining ethics and regulatory approval for the trials, particularly for early-stage trials.
Regulators including the United States Federal Drug Administration (FDA) have been at the forefront of advocating for the use of non-invasive assessment techniques for liver fibrosis over invasive approaches (including liver biopsy) and have actively supported the development of new and improved non-invasive techniques. The FDA has highlighted that the lack of validated non-invasive liver fibrosis assessment methods remains an unmet challenge including for the development of a range of drugs for NASH and NAFLD.[vii]
Current non-invasive approaches to liver fibrosis
Several techniques exist that purport to indicate the presence of liver fibrosis utilising non-invasive or less-invasive procedures than a liver biopsy. These include serum blood tests, ultrasound, and magnetic resonance elastography (MRE) methods and quantitative MRI methods. While these methods may provide information on the presence of liver fibrosis (particularly advanced fibrosis), they have various unresolved issues inhibiting the accurate and reliable detection of fibrosis. In addition, these techniques are typically confounded by the presence of other factors in the liver including inflammation, iron level (or content), subcutaneous and liver fats, ascites, and others.
Resonance Health’s initial proof of concept success
Resonance Health has completed an initial proof-of-concept study of a novel non-invasive imaging-analysis technique to identify and assess liver fibrosis (Initial Proof of Concept). The Initial Proof of Concept study, which was conducted on a patient and control group of 30 subjects, indicates a strong capability to predict the absolute presence of liver fibrosis within the study population and it forms the basis of the invention which is the subject of the provisional patent application (see here).
The Initial Proof of Concept and the provisional patent application is based on a novel approach devised and developed by a team led by Chief Scientific Officer, Dr. Wenjie Pang. Dr. Pang is a PhD physicist and has worked on non-invasive liver imaging technologies throughout his career including FerriScan® which successfully obtained regulatory clearances and is widely published, and now regarded by numerous clinicians as the global gold standard for liver-iron-concentration assessment.
Next steps & KOL engagement
Based on the extremely promising Initial Proof of Concept study results Resonance Health is undertaking an accelerated extended proof-of-concept study with an expanded study population (Extended Proof of Concept). Engagement with globally recognised clinical key opinion leaders (KOLs) has commenced, with all those contacted expressing strong interest in collaborating on developing the new technology.
Extended Proof of Concept
The objectives of the Extended Proof of Concept are to confirm the results of the Initial Proof of Concept, to further refine the study predictive models and to further assess the performance of predictive models and their capacity to distinguish between differing fibrosis grades (see diagram above). The duration of the Extended Proof of Concept is estimated to be 6-12 months. The Company is formally engaging clinical KOLs to secure the study subjects required for the Extended Proof of Concept.
Subject to the outcomes of the Extended Proof of Concept, the Company will collaborate with a much larger group of clinical KOLs, and pharmaceutical companies, who have expressed strong interest in the results of the Initial Proof of Concept and a desire to participate in collaborative opportunities should they arise. The Company also intends to engage with global regulators including the FDA as the Extended Proof of Concept progresses.
[i] Cotter TG, Rinella M (2020) Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 158(7):1851–1864. https://doi.org/10.1053/j.gastro.2020.01.052 PMID: 32061595
[ii] Nonalcoholic fatty liver disease and cardiovascular diseases phenotypes, Glandomenico Bisaccia et al, SAGE Open Medicine Volume 8: 1-15, 21 May 2020.
[iii] Blach S, Zeuzem S, Manns M, Altraif I, Duberg A, Muljono DH, et al. (2017) Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2(3):161–176. https://doi.org/10.1016/S2468-1253(16)30181-9 PMID: 28404132
[iv] Seto WK, Lo YR, Pawlotsky JM, Yuen MF (2018) Chronic hepatitis B virus infection. Lancet 392 (10161):2313–2324. https://doi.org/10.1016/S0140-6736(18)31865-8 PMID: 30496122
[v] Prof. John Olynyk, see Resonance Health (ASX RHT) ASX release dated 24 November 2021.
[vi] GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–171
[vii] Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment – Guidance for Industry, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). https://www.fda.gov/media/119044/download