Resonance Health Ltd (ASX: RHT) (“Resonance Health” or “Company”) announces that it has received 510(k) clearance from the US Food and Drug Administration (“FDA”) for HepaFat-AI, a medical device (software) that is fully automated and uses artificial intelligence (“AI”) to assess liver fat.
The 510(k) clearance allows Resonance Health to market HepaFat-AI for commercial distribution in the United States of America.
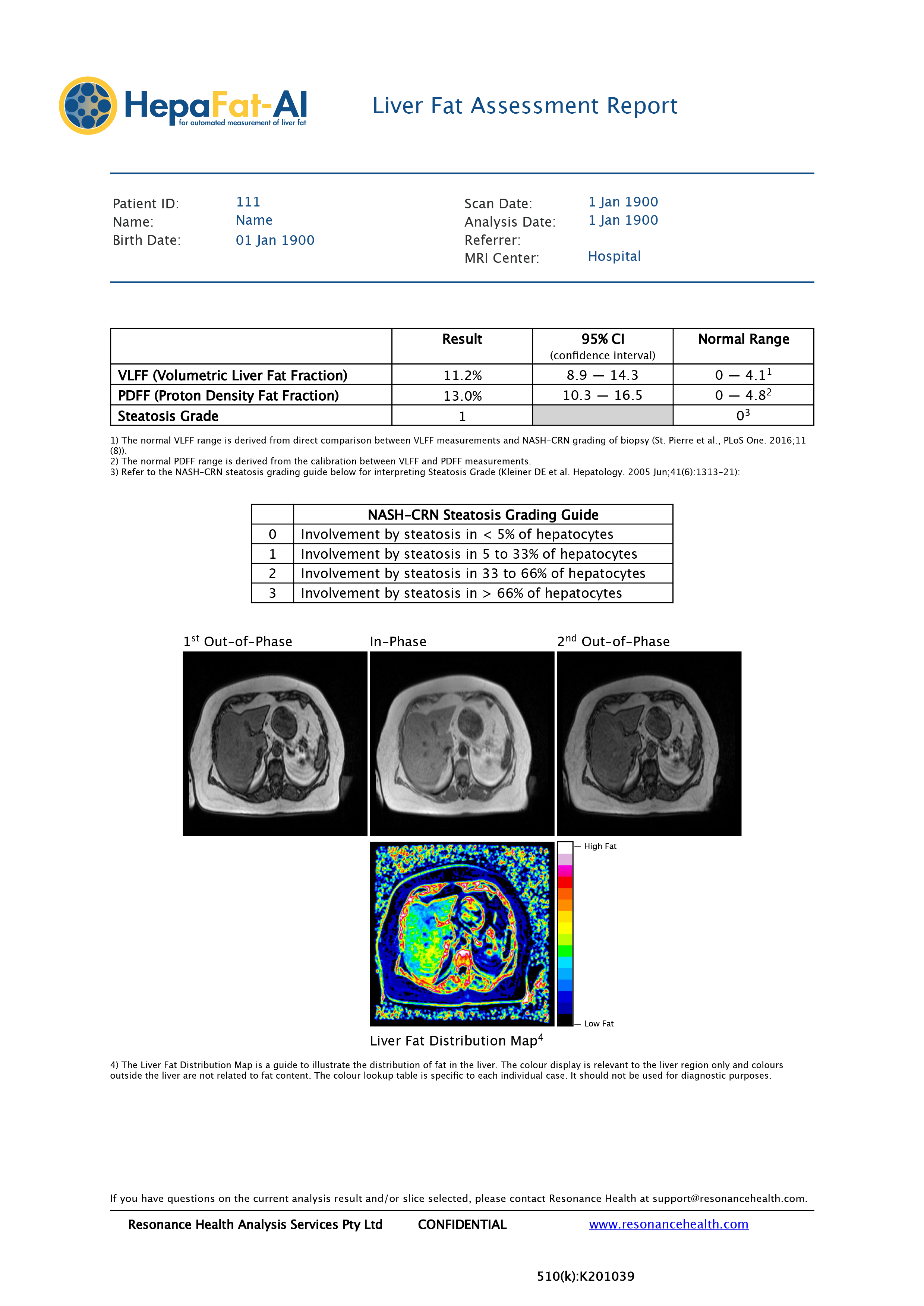
HepaFat-AI assesses the volumetric liver fat fraction (‘VLFF’), proton density fat fraction (‘PDFF’), and steatosis grade in individuals with confirmed or suspected fatty liver disease. When interpreted by a trained physician, the HepaFat-AI results (see example of HepaFat-AI report at Annex A) can be used to monitor liver fat content in patients undergoing weight loss management, and to aid in the assessment and screening of living donors for liver transplant.
HepaFat-AI automatically analyses magnetic resonance imaging (“MRI”) datasets to assess liver fat in patients, providing doctors with a comprehensive, multi-metric tool for use in the assessment of fatty liver. HepaFat-AI produces a patient report for clinician interpretation within seconds, which in addition to reporting the above, includes a liver fat distribution map that illustrates the distribution of fat in the liver (Figure 1. below).
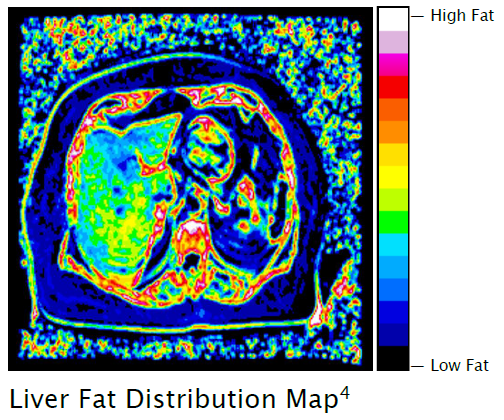
Figure 1:Sample Liver Fat Distribution Map. Full report example can be seen at the bottom of this page.
HepaFat-AI may be useful for physicians when assessing and monitoring patients with suspected fatty liver disease. It is estimated that 24-30% of the global population has Non-Alcoholic Fatty Liver Disease (NAFLD)1, which equates to 1.8 – 2.3 billion people. Of these, it is estimated that 20%, or up to 468 million people, will also develop non-alcoholic steatohepatitis (NASH), the most severe form of NAFLD where inflammation can cause liver damage and fibrosis.
If the prevalence of NAFLD continues to rise in line with the global obesity epidemic, it is predicted that the healthcare burden of NAFLD over the next 10 years could increase to $1.005 trillion in the USA alone, and €334 billion across Germany, France, Italy, and the United Kingdom2.
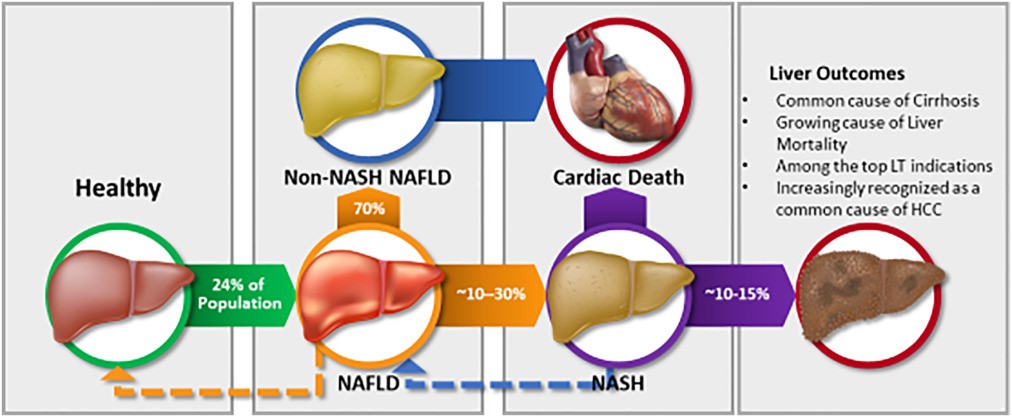
Figure 2: A simplified version of the natural history of NAFLD and NASH3
There are a number of ways to measure liver fat currently. These include PDFF methods [MRI (magnetic resonance imaging) or MRS (magnetic resonance spectroscopy)], assessment using liver biopsy, and the calculation of a steatosis grading (the grading of the percentage of fat that has accumulated in hepatocytes for a patient).
Globally, there are a number of PDFF techniques in use currently. PDFF methods vary considerably and are dependent on the specific acquisition parameters used. Direct comparison of results between scanner types and image acquisition methods is therefore extremely difficult.
The ability to perform direct comparisons longitudinally and across scanner types is one of the key features of HepaFat-AI, which delivers a standardised, fully reproducible PDFF assessment for directly comparable results regardless of scanner make or model. HepaFat-AI also calculates a steatosis grade in addition to the VLFF and PDFF result. This steatosis grading has traditionally been a time-consuming method undertaken by highly trained histopathologists and is now a task Resonance Health has fully automated.
HepaFat-AI can be deployed either in the cloud or on premises and can be integrated directly into radiology workflows for faster pathways to established customer bases. The Company intends to actively pursue multiple routes to market for HepaFat-AI.
Dr Martin Blake, Chairman of RHT, says “This is a great milestone in the Company's history and a magnificent achievement in the field of quantitative MRI.”
Resonance Health CEO, Alison Laws says “The use of artificial intelligence in medical image review and assessment has the ability to support and transform radiology via the delivery of rapid, reproducible results whenever needed. HepaFat-AI, the Company’s second AI-based medical device to gain clearance from the FDA, will enable radiologists to report three key parameters (VLFF, PDFF, and steatosis grade) in patients in this significant and ever-increasing addressable market.
The clearance from the FDA to assess and report all three metrics in HepaFat-AI patient reports is an outstanding result for the Company and an outstanding result for the product itself.”
Additional information on HepaFat-AI and its application in confirmed or suspected fatty liver disease is available on our product-specific website, www.hepafat.com.
Further updates will be provided in due course on the Company’s work towards obtaining Australian Therapeutic Goods Administration (TGA) regulatory clearance and CE Mark (Europe) for HepaFat-AI.
Authorised by:
This announcement has been authorised for release in accordance with the delegated authority of the Board of Resonance Health Limited.
- Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clinics in Liver Disease. 2016;20:205-214
- Younossi, Z. M. et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64, 1577–1586 (2016).
- Younossi, Z.M. (2018), The epidemiology of nonalcoholic steatohepatitis. Clinical Liver Disease, 11: 92-94. doi:10.1002/cld.710