HepaFat-Scan is a non-invasive MRI-based solution for measuring the volume fraction of fat in liver tissue. HepaFat-Scan is provided as an image analysis service; with analysis and reporting provided from the Resonance Health ISO 13485 certified Service Centre via our Quality Assured Service Delivery model.
The HepaFat-Scan software is the first and only method with regulatory clearances (USA – FDA, Europe – CE Mark, Australia – TGA) for quantitative measurement of liver fat that has been clinically validated against independent measurements of the volume fraction of fat in liver biopsy specimens in a clinical study.
HepaFat-Scan reports volumetric fraction of fat in the liver, a measure that can be directly compared to a volumetric biopsy fat fraction measurement.
HepaFat-Scan is now available for routine clinical use and for use in pharmaceutical company clinical trials.
HepaFat-Scan Key Features: 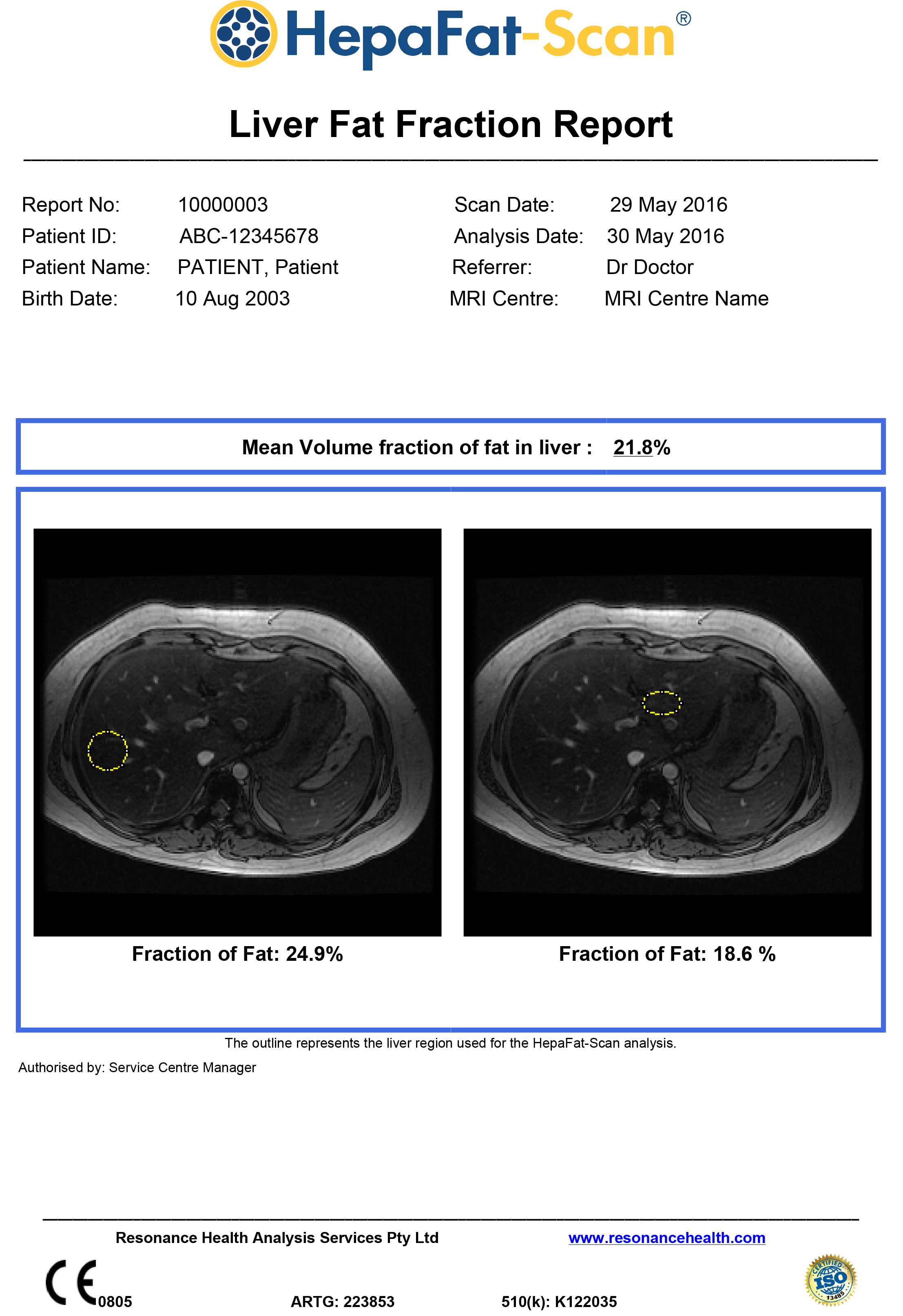
- HepaFat-Scan is clinically validated against biopsy measurements of liver fat (59 cases)
- Clinically insignificant bias compared with biopsy (1.4%)
- Image analysis and reporting is performed at a central ISO 13485 certified Service Centre
- HepaFat-Scan has international regulatory clearance and approvals (USA, Europe, Australia)
- Results are available with a targeted turnaround time of two business days
- HepaFat-Scan is non-invasive, requires no contrast agents and has a scan time of approximately two minutes
- Single breath hold scan
- Large liver region >300 times larger than biopsy, reducing sampling error
- Results are accurate, reliable and reproducible over time and between MRI centres and models of scanner – making it suitable for clinical trials
- There is no requirement for customers to purchase new software or hardware, resulting in minimal training requirements
- High sensitivity and specificity when compared against histopathological assessment of steatosis
- Suitable for 1.5 and 3 Tesla MRI scanners
To see more information, view the HepaFat-Scan Fact Sheet.
Why Use HepaFat-Scan to Measure Liver Fat
HepaFat-Scan is quick, easy and painless, with an MRI scan time of only two minutes. HepaFat-Scan does not require any contrast agents and being MRI based there is no risk from radiation.
Histopathological assessment of a patient’s liver fat levels from a liver biopsy is commonly considered the gold standard for clinical assessment of liver fat. However, it is subjective, has a poor reproducibility and significant sampling error and suffers from the risks associated with an invasive procedure.
Ultrasound, while widely available, only has a sensitivity of approximately 70% and this is further reduced in obese patients. It is additionally hampered by factors such as fibrosis, edema and extrahepatic adipose tissue.
CT is also a widely available imaging modality that evaluates hepatic steatosis indirectly based on hepatic x-ray attenuation. Several factors other than fat (e.g. iron, copper, glycogen, fibrosis, edema, ingestion of drugs such as amiodarone and gold) affect CT attenuation values, resulting in unavoidable errors in fat quantification and low sensitivity for mild-moderate steatosis. Moreover, as CT relies on ionizing radiation, it is not suitable for use in children and for longitudinal monitoring of adults with liver fat. MR has also been used for quantitative assessment of fat and is widely accepted as superior compared to ultrasound and CT.
HepaFat-Scan is used in conjunction with a specific MRI data acquisition protocol that is a variation of the Dixon Method. Many MR fat quantification methods such as Magnetic Resonance Spectroscopy (MRS) and other Dixon based imaging methods report the fat:water proton ratio. This ratio is not directly comparable to a fat measurement from a liver biopsy specimen as it neither represents the percentage of parenchyma involved with fat nor the volume fraction of fat in the biopsy. Resonance Health has addressed this issue with HepaFat-Scan.
HepaFat-Scan measures the volumetric fat fraction of the liver tissue. This is a measure that is directly comparable to what can be seen and measured in a biopsy specimen; that is the fraction of the tissue that is observed to be fat. Hence, HepaFat-Scan can be used as a surrogate for liver biopsy measurement of hepatic steatosis.
Potential Clinical Applications
Gastroenterology and Hepatology Applications
- Initial workup for NAFLD diagnosis and for patient education and counselling.
- Liver fat analysis on patients already being screened or monitored for fibrosis or cirrhosis. Recent research indicates that patients with NAFLD and NASH can develop liver cancer without progressing through cirrhosis.
Surgical Applications
- Scanning of patients with liver disease or liver cancer to provide guidance with treatment planning. The liver percent fat may help to determine how much liver can be safely resected or to determine whether to use a surgical or non-surgical treatment. A high level of liver fat may have a detrimental outcome on liver surgery.
- Screening for suitability of living donors for liver transplants by assisting in determining the viability of the liver
- Pre and post operative analysis of bariatric patients to track clinical outcomes.
Applications by Primary Care Physicians
- HepaFat-Scan may be useful to screen patients prior to prescribing known hepatotoxic medications.
- Monitoring of patients undergoing an intervention (e.g. limiting alcohol consumption or weight loss)
- Monitoring of patients prescribed medications known to induce steatosis
HepaFat-Scan Clinical Validation
Resonance Health has completed a clinical study comparing 59 patient measurements of liver fat fraction using HepaFat-Scan and stereological analysis of liver biopsy sections. Stereology is a computer assisted analysis of each biopsy specimen to quantify the volume fraction of the biopsy that is fat. The HepaFat-Scan results can be directly compared against the biopsy measurements as both report a volume percentage of fat. The results of the comparison are illustrated in the figure below. A linear relationship between the two sets of measurements was found with a clinically insignificant bias (1.4% by Bland Altman analysis) between the two types of measurement.
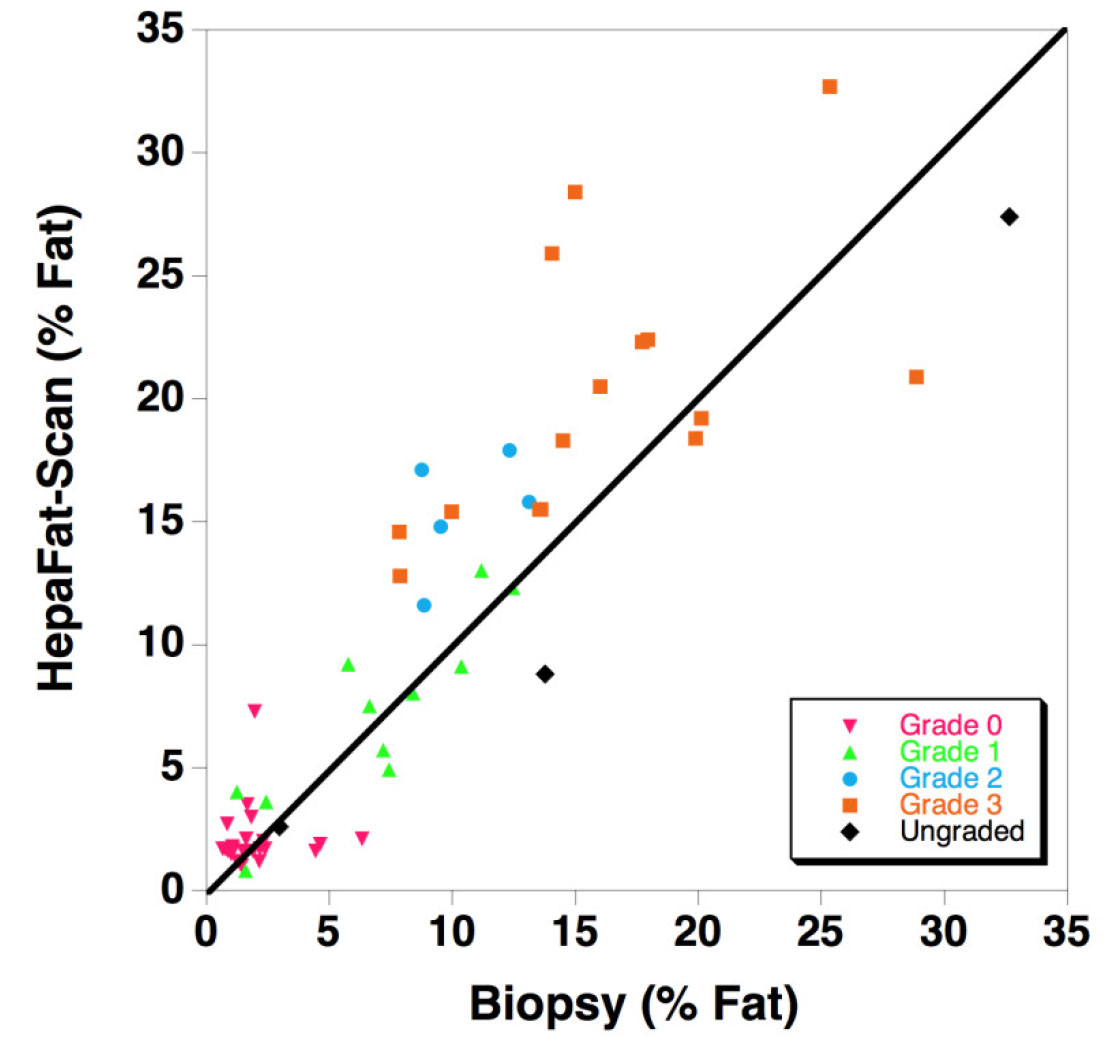
The figure displays a comparison of HepaFat-Scan measurements of liver fat fraction with those measured from biopsy sections using stereology (n=59). The solid line is the line of equivalence. Symbols are colour/shape-coded to indicate the NAS CRN steatosis grade assessed by a histopathologist according to the below table using the Nonalcoholic Steatohepatitis Clinical Research Network grading scheme [Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease, Hepatology, June 2005, Kleiner et al]:
- Grade 0: < 5% parenchymal involvement by steatosis
- Grade 1: 5% – 33% parenchymal involvement by steatosis
- Grade 2: >33%-66% parenchymal involvement by steatosis
- Grade 3: >66% parenchymal involvement by steatosis
All grades of steatosis were represented in the study [Grade 0 (47%); Grade 1 (13%); Grade 2 (9%); Grade 3 (30%)]. The patients in the study had a range of aeitiologies including autoimmune hepatitis (n=3), alcoholic liver disease (n=2), chronic viral hepatitis B/C (n=16), non-alcoholic fatty liver disease (n=10), nonalcoholic steatohepatitis (n=17), primary sclerosing cholangitis (n=4).
| Sensitivity and Specificity of HepaFat-Scan | |||
|---|---|---|---|
| Steatosis Grades |
Grade 0 vs 1-3 (≥5%) |
Grades 0-1 vs 2-3 (≥33%) |
Grades 0-2 vs 3 (≥66%) |
|
Sensitivity (%) (95% conf interval) |
96.77 (83-100) | 100.00 (83-100) | 100.00 (78-100) |
|
Specificity (%) (95% conf interval) |
96.00 (80-100) | 94.44 (81-99) | 87.80 (74-96) |
| AUROC (standard error) | 0.963 (0.032) | 0.996 (0.005) |
0.971 (0.019) |
If you wish to use the HepaFat-Scan service or would like to know more about HepaFat-Scan, please send an email to info@resonancehealth.com